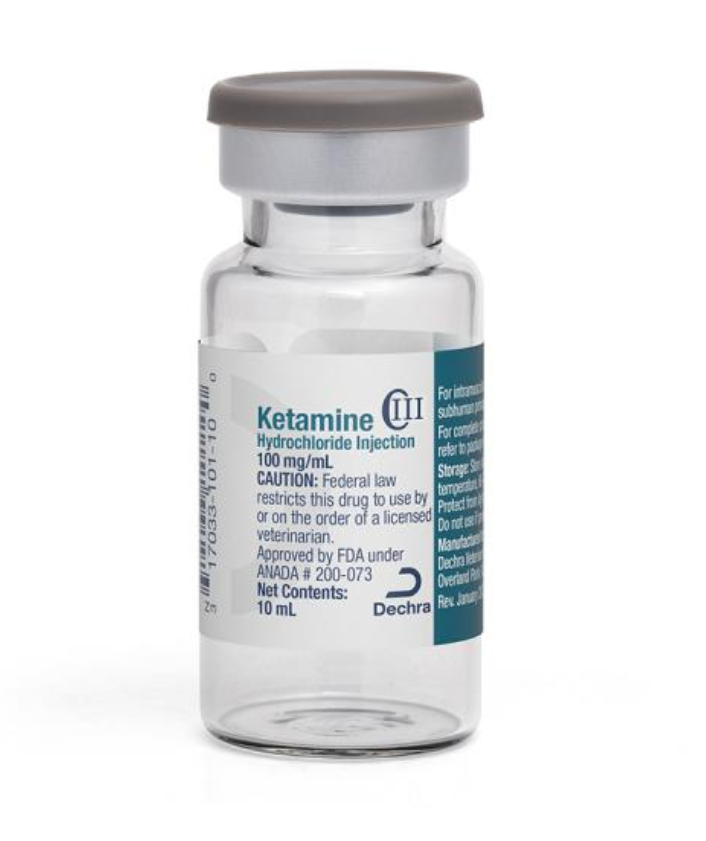
Ketamine Hydrochloride Injection
Federal law restricts this drug to use by or on the order of a licensed veterinarian. As with all drugs, side effects may occur. Ketamine Hydrochloride Injection is contraindicated in cats and subhuman primates suffering from renal or hepatic insufficiency. To reduce the incidence of emergence reactions, animals should not be stimulated by sound or handling during the recovery period. Apnea, respiratory arrest, cardiac arrest and death have occasionally been reported with ketamine used alone, and more frequently when used in conjunction with sedatives or other anesthetics. Respiratory depression may occur following administration of high doses of Ketamine Hydrochloride Injection. If at any time respiration becomes excessively depressed and the animal becomes cyanotic, resuscitative measures should be instituted promptly. In the cat, myoclonic jerking and/or mild tonic convulsions can be controlled by ultrashort‐acting barbiturates which should be given to effect. The barbiturates should be administered intravenously at a dose level of one‐sixth to one‐fourth the usual dose for the product being used.
Ketamine acts by blocking receptors in the brain called NMDA receptors, which are involved in inhibitory processes. The blockade of these receptors leads to increased mTOR pathway signaling, increased AMPA receptor activation, and increased development and efficacy of synapses in the brain, leading to an anti-depressant effect. Ketamine nasal spray may be used for the treatment of depression that does not respond to other therapies.
- This product is qualified as a Certified Reference Material (CRM) that has been manufactured and tested to meet ISO/IEC 17025 and ISO 17034 international standards
- This CRM has been characterized by metrologically valid procedures and may be used as a quantitative analytical reference standard
- Certificate of analysis provides the certified property values and the associated uncertainties, including a statement of metrological traceability for the certified values
- Possession of a DEA Controlled Substance registration is not necessary nor is a DEA 222 form required to purchase this product
- Bulk material is available for qualified institutions; please contact our sales department for pricing
Ketamine (hydrochloride) (CRM) (Item No. 19389) is a certified reference material categorized as an arylcyclohexylamine. Ketamine is a dissociative anesthetic that has been abused recreationally.1,2,3 Ketamine (CRM) (Item No. 19389) is provided as a DEA exempt preparation. This product is intended for research and forensic applications.
Information gained from the capture and handling of free-ranging animals helps advance the understanding of wildlife ecology and aids in crafting wildlife management policy. Unfortunately, animals can experience increased levels of stress, injuries, and death resulting from captures.1 Fortunately, some of the above information has also helped immeasurably in the development of drug formulations that actually decrease the risks associated with capture and chemical immobilization. Since the advent of modern chemical immobilization techniques, developing drug formulations that address both the physiological attributes of individual species as well as the effects of the varied and numerous conditions encountered in the field have been among the chief objectives of wildlife veterinarians, wildlife managers, researchers and the veterinary pharmaceutical industry.

Reviews
There are no reviews yet.